
Lithium battery structure and principle interpretation
2022-12-22 18:001. Basic structure of lithium battery
Main materials: positive electrode, negative electrode, electrolyte, separator
Structure: round, square (prismatic); laminated, coiled
Form: polymer (soft packaging), liquid lithium ion (steel shell)
2. Working principle of lithium battery
Positive electrode material (anode material): LiMn2O4,
negative electrode material (cathode material): graphite
When charging, Li+ in the positive electrode and Li+ in the electrolyte gather towards the negative electrode, which forms electrons, and are reduced to Li and embedded in the carbon material of the negative electrode. During discharge, the Li embedded in the carbon material of the negative electrode loses electrons and enters the electrolyte, and the Li+ in the electrolyte moves to the positive electrode.
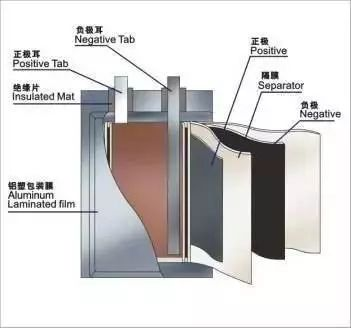
3. Composition principle of lithium battery
①Positive structure
LiMn2O4 (lithium manganese oxide) + conductive agent (acetylene black) + binder (PVDF) + current collector (aluminum foil) positive electrode
②Negative electrode structure
Graphite + conductive agent (acetylene black) + binder (PVDF) + current collector (copper foil) negative electrode
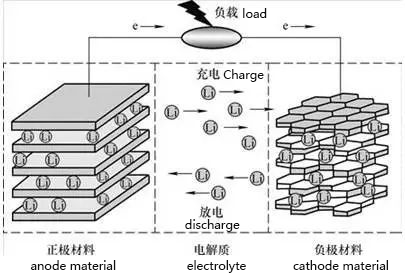
4. charging process
The power supply charges the battery. At this time, the electrons e on the positive electrode run from the external circuit to the negative electrode, and the positive lithium ions Li+ "jump" into the electrolyte from the positive electrode, "climb" through the winding holes on the separator, and "swim" "When it reaches the negative electrode, it combines with the electrons that have already run over.
The reaction on the positive electrode is LiMn2O4 ==Li1-xMn2O4+Xli++Xe (electron)
The reaction on the negative electrode is 6C+XLi+Xe==LixC6
5. discharge process
When the battery is discharged, the electrons e on the negative electrode run from the external circuit to the positive electrode, and the positive lithium ion Li+ "jumps" from the negative electrode into the electrolyte, "climbs" through the small tortuous hole on the separator, and "swims" to reach the positive electrode is combined with the electrons that have already run over.
The reaction that occurs on the positive electrode is Li1-xMn2O4+xli++xe (electron) ==LiMn2O4
The reaction on the negative electrode is LixC6 == 6C+xLi+xe
